Melanoma In Situ of Leg – Mapping Excision Margins
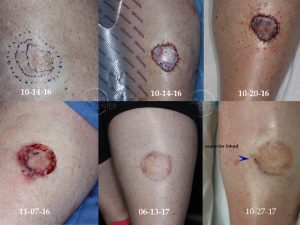
The vexing problem of positive margins after excision of melanoma in situ has many solutions. In this article, we summarize them and rank them in the order of efficacy.
Melanoma in situ excision margin guidelines range from the older 5 mm margin to the aggressive 10 mm margin of resection. 7 mm seems to be the median contemporary recommendation for margin of excision around the visible borders. But these are just guidelines that a real melanoma in situ can easily confound as many of the cases presented in our case studies have demonstrated. Margins of larger lesions in dyschromic pigmented skin, such as lentigo malignas, are particularly hard to define and to clear surgically. Surgical clearance goals are cure with minimal normal tissue sacrifice. Therefore, pre-operative mapping of MIS margins serve as the necessary tool for conservative curative surgery in larger melanoma in situ lesions or in those with poorly defined borders.
Wood’s light first, described in 1903, is an ultraviolet light that takes advantage of melanin’s UV absorption property. It is an easy and inexpensive modality. Its limitation is superficial epidermal melanin visualization with no dermal melanin visualization. Jeneby et. al (2001) found that Wood’s light guided punch biopsies of melanoma in situ and superficially invasive lentigo maligna melanoma cleared the tumor 67% of the time. The other 33% of the time additional 1-3 mapping biopsies were required to clear the tumor that extended subclinically beyond visual and Wood’s light margins. Robinson (2004) quantified the advantage of the Wood’s light over clinical assessment as 0.2 – 0.4 cm.
Dermoscopy and its subset of digital epiluminescence microscopy (DELM) are considered a more accurate visualization modality than a Wood’s light. Robinson (2004) as above quantified DELM as 0.2 – 0.3 cm better than a Wood’s light. Histologic clearance of lentigo maligna lesions required a 5 mm margin beyond the DELM margins.
Reflectance confocal laser microscopy (RCM or CLM) imaging of the skin is even more accurate. Guitera et. al (2013) quantified the benefit of RCM in large facial partially pigmented melanomas in situ and lentigo maligna melanomas. RCM found margins at least 5 mm beyond those identified by dermoscopy. Overall, RCM resulted in a major change in treatment in 73% of patients in the study. However, reflectance confocal microscopy still remains a cumbersome and impractical skin imaging modality. The image acquisition time of an area of 8 x 8 mm is upwards of 20 minutes. Still at that point, an RCM expert requires 5 minutes to read just one image area.
The gold mapping standard for melanoma in situ and lentigo maligna melanoma remains punch biopsies. Dengel et. al (2008) nicely describes the process of serial mapping biopsies using a 2 mm punch. Each series of biopsies is placed 1 cm away from the last positive biopsy or margin. This cycle is repeated until biopsies find more abnormal melanocytes. At that point surgical excision is performed.
Mohs surgery for melanoma in situ has a role in the management of these tumors. However, there are several limitations with this technique. The first is the quality control and expertise necessary. Simple hematoxylin and eosin (H&E) staining of frozen sections is used, but immunohistochemistry staining with MART-1, melan A, S100, or HMB-45 has been shown to be more accurate. Employment of these stains requires a high volume of histology lab utilization as well as expertise of technicians and Mohs surgeons. The second limitation is the difficulty with margin interpretation even with immuno stains. Confirmation of margins clearance is often necessary with permanent H&E staining of margins and dermatopathologist interpretation.
References
1.Chen, C. S. J., Elias, M., Busam, K., Rajadhyaksha, M., & Marghoob, A. A. (2005). Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. British Journal of Dermatology, 153, 1031-1036.
Dengel, L., Turza, K., Noland, M. B., Patterson, J. W., & Slingluff, C. L. (2008). Skin mapping with punch biopsies for defining margins in melanoma: when you don’t know how far to go. Annals of Surgical Oncology, 15(11), 3028-3035.
Guitera, P., Moloney F. J., Menzies, S. W., Stretch, J. R., Quinn, M. J., Hong, A.,…Scolyer, R. A. (2013). Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatology, 149(6), 692-698.
Jeneby, T. T., Chang, B., & Bucky, L. P. (2001). Ultraviolet-assisted punch biopsy mapping for lentigo maligna melanoma. Annals of Plastic Surgery, 46(5), 495-500.
Robinson, J.K. (2004). Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Archives of Dermatology, 140, 1095-1100.
Paraskevas, L. R., Halpern, A.C., & Marghoob, A. A. (2004). Utility of wood’s light: five cases from a pigmented lesion clinic. British Journal of Dermatology, 152, 1039-1044.
